Total Alkalinity & Calcium Hardness - Alkavis Comparator Tablets (Pack Of 250)
Total Alkalinity & Calcium Hardness - Alkavis Comparator Tablets (Pack Of 250)
Total Alkalinity and Calcium hardness, Alkavis comparator tablets, pack of 250.
Log in to see prices
Couldn't load pickup availability
Share
Description
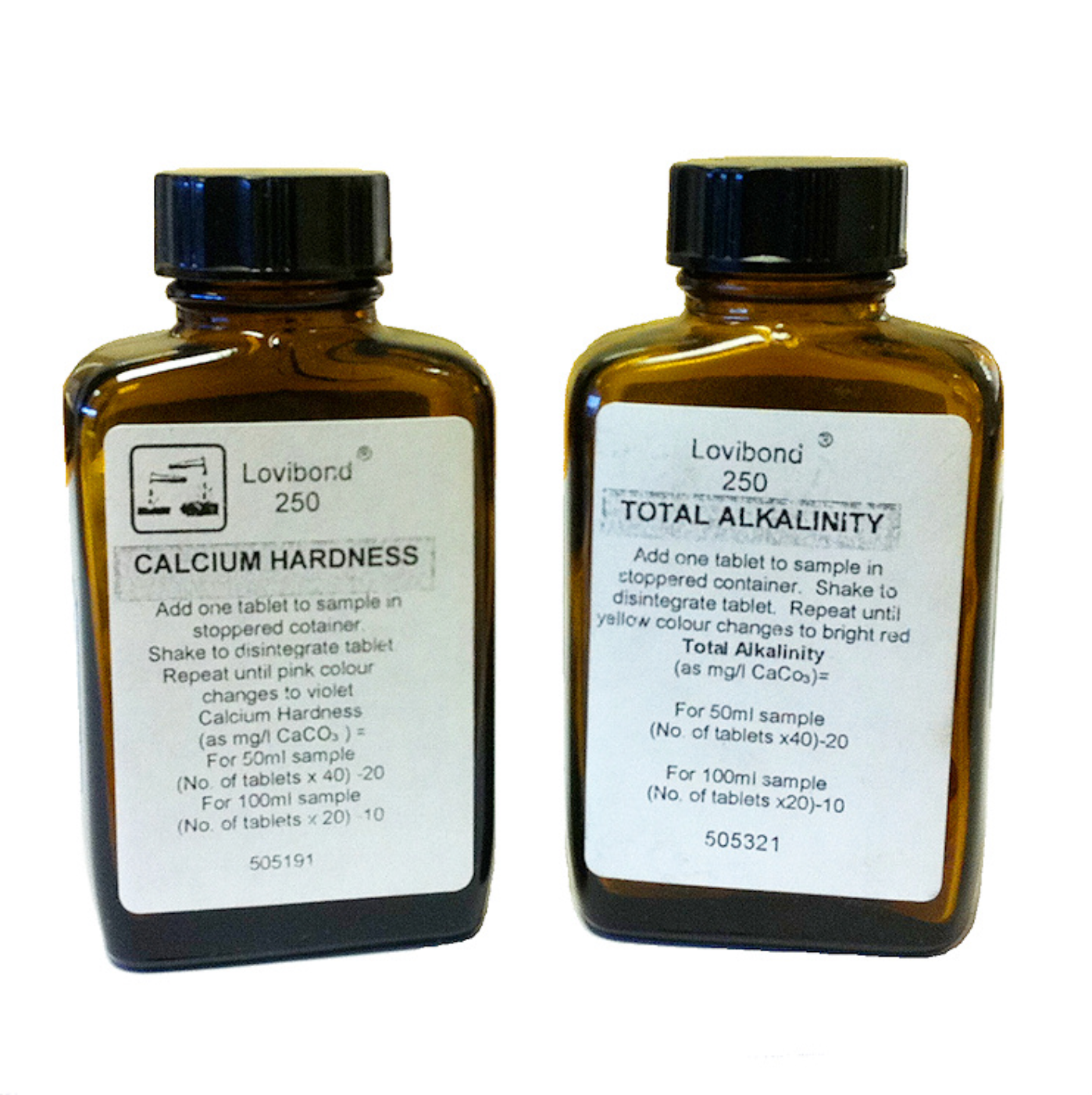
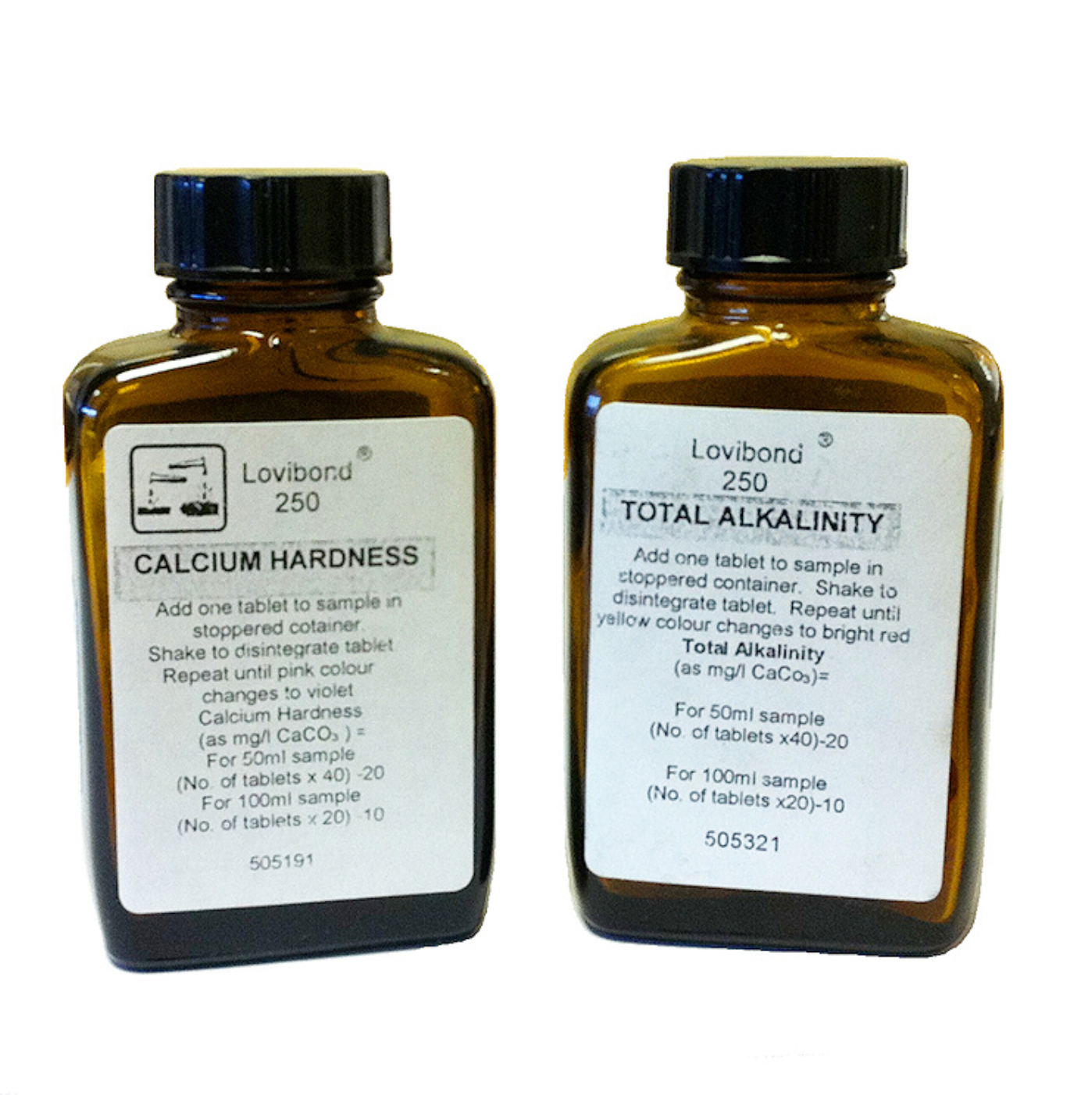
Calcium Hardness tablets - 250 tablets to be used in conjunction with the 50ml or 100ml shaker bottles. These tablets measure calcium hardness as mg/l - simply add one tablet to your sample, stopper and shake to disintegrate tablet. Repeat until pink colour changes to violet.
Calcium Hardness can then be calculated as:
50ml sample container = number of tablets added multiplied by 40 then minus 20 to give you your Calcium Hardness reading. i.e. 6 tablets added, so (6 x 40) - 20 = 220 mg/l
100ml sample container = number of tablets added multiplied by 20 then minus 10 to give you your Calcium Hardness reading. i.e. 11 tablets added, so (11 x 20) - 10 = 210 mg/l
As you can see from the above examples it is more tablet efficient to use a 50ml stoppered sample of water for testing.
Total Alkalinity - 250 Tablets - Add one tablet to sample in a stoppered container. Shake to disintegrate tablet. Repeat until yellow colour changes to bright red.
Total Alkalinity can then be calculated as:
50ml sample container = number of tablets added multiplied by 40 then minus 20 to give you your Total Alkalinity reading. i.e. 6 tablets added, so (6 x 40) - 20 = 220 mg/l
100ml sample container = number of tablets added multiplied by 20 then minus 10 to give you your Total Alkalinity reading. i.e. 11 tablets added, so (11 x 20) - 10 = 210 mg/l
As you can see from the above examples it is more tablet efficient to use a 50ml stoppered sample of water for testing.
What is Calcium Hardness?
Most of the hardness of your water supply is dissolved Calcium salts due to the water having arisen in Chalk or Limestone areas. Magnesium salts also tend to be grouped with Calcium in this description. Some hardness is good for your pool otherwise Calcium from sources such as tile grout will be dissolved. Conversely, too much causes scaling problems.
Calcium hungry?
If water is very soft i.e. it contains little dissolved Calcium from a hard water source, it will then dissolve Calcium from other sources such as concrete and tile grout. The use of Calcium Chloride (Water Hardness Increase) will counteract this, as may the use of Calcium Hypochlorite as a sanitiser
What is Total Alkalinity?
This is a measure of all the alkaline materials in the pool water. In the pH range found in pool waters, the alkalinity is likely to be present in the form of Sodium Bicarbonate. At the correct level, the pH will not alter rapidly as the water is said to be buffered. If the total alkalinity is below 80 p.p.m. then the water will be insufficiently buffered and if it is above 200 p.p.m it will be excessively buffered. In either case the effect of the addition of chemicals will be difficult to control.